- Comment
- Open access
- Published:
Research Buddy partnership in a MD–PhD program: lessons learned
Research Involvement and Engagement volume 9, Article number: 4 (2023)
Abstract
Background and aims
There is increasing recognition of the importance of patient involvement in research. In recent years, there has also been growing interest in patient partnerships with doctoral studies students. However, it can be difficult to know where to start and how to go about such involvement activities. The purpose of this perspective piece was to share experiential insight of the experience of a patient involvement program such that others can learn from this experience.
Body
This is a co-authored perspective piece centred on the experience of MGH, a patient who has had hip replacement surgery, and DG, a medical student completing a PhD, participating in a Research Buddy partnership over the course of over 3 years. The context in which this partnership took place was also described to facilitate comparison with readers’ own circumstances and contexts. DG and MGH met regularly to discuss, and work together on, various aspects of DG’s PhD research project. Reflexive thematic analysis was conducted on reflections from DG and MGH regarding their experience in the Research Buddy program to synthesise nine lessons which were then corroborated with reference to published literature on patient involvement in research. These lessons were: learn from experience; tailor the program; get involved early; embrace uniqueness; meet regularly; build rapport; ensure mutual benefit; broad involvement; regularly reflect and review.
Conclusions
In this perspective piece, a patient and a medical student completing a PhD reflected upon their experience co-designing a Research Buddy partnership within a patient involvement program. A series of nine lessons was identified and presented to inform readers seeking to develop or enhance their own patient involvement programs. Researcher-patient rapport is foundational to all other aspects of the patient’s involvement.
Plain English summary
The importance of patient involvement in research is gaining recognition. Existing research centres, as well as those that are just getting started, need to find their own way to involve patients and community members. However, learning from the experience of others is crucial to ensure every effort is made to do this in a fruitful way. Therefore, we aimed to share our experience and provide a list of lessons learned to help other researchers and patients get started and work together effectively. Our research centre developed a framework for involving patients in joint replacement research. Part of this framework is a ‘Research Buddy’ program, where a research student partners with a patient so that the research they conduct is more relevant and applicable to the target population. In our case, the research student partnered with someone who had a hip replacement to develop and test a questionnaire for an interview study about artificial intelligence in shared decision-making. The student and patient worked together and wrote this perspective piece outlining nine lessons so readers can learn from their experience of this program. The lessons were: learn from experience, tailor the program, get involved early, embrace uniqueness, meet regularly, build rapport, ensure mutual benefit, broad involvement, regularly reflect and review. People interested in starting, or improving, their own patient involvement activities can learn from our experience. These lessons will need to be adapted to fit the purpose and unique situation of other researchers and patients who have different needs and circumstances.
Introduction
Background
There is growing recognition of the importance of patient and public involvement and engagement in research [1, 2]. However, it can be difficult to know where to begin and how to go about it. Sharing experiential knowledge is critical for different groups seeking to develop and deploy their own programs and strategies [3]. This is because a one-size-fits-all approach will not address the culture and context of the unique settings in which these programs take place [4,5,6,7]. Rather, strategies should be tailored to each unique setting [8]. An in-depth description of the experience of the patient involvement program in a particular context, including the nature of the program and the challenges overcome, provides others with ideas and examples they can modify to suit their own needs [1, 3, 9]. These other groups can read about the experience of others and possibly avoid some pitfalls while harnessing the strengths of the program. This is not intended to restrict creativity nor to provide a prescriptive roadmap, but rather to provide an experiential account of the nature of engagement in a particular setting and offer suggestions for ways in which engagement can take place.
In recent years, there has also been growing interest in efforts to involve patients in doctoral studies [5, 10,11,12,13,14,15,16,17]. This presents a unique opportunity to instil recognition of the importance of patient involvement early in the career of researchers and equip them with the skills and experience to facilitate meaningful involvement. It simultaneously offers patients an opportunity to partner with researchers in this formative stage of their career as they develop their research interests and approach to research.
Aims and rationale
The aim of this perspective piece was for the first two members (DG and MGH) of a Research Buddy partnership (described below in the ‘Context’ section) to critically reflect on their experience of this involvement activity throughout the course of a MD–PhD program.
Through this reflective process, lessons learned through experience were consolidated such that they can be of benefit to others seeking to learn from this experiential insight as they establish or improve their own patient involvement programs [1, 3, 18,19,20]. We also provided a detailed description of the context in which this Research Buddy partnership took place, to provide readers with a more complete understanding of the way in which this context influenced the partnership such that readers can consider the nature of their own unique situation and how this might impact upon patient involvement efforts [21].
Overview of structure of this perspective piece
Definitions.
Theoretical Underpinnings.
Context.
Methods.
-
Method of Patient Involvement throughout MD–PhD program
-
Method of reflection in this perspective piece
Findings—Lessons Learned.
Discussion
Conclusions
Definitions
The body of literature in the patient involvement space is fraught with complex and nuanced terminology [22]. Therefore, the purpose of this section is to clarify the terminology and definitions used. Influential stakeholder involvement literature, as well as recent publications, were reviewed to select widely-used and functionally relevant terminology for this perspective piece [1, 22,23,24,25,26,27,28,29,30,31,32,33]. The other critical aspect of the rationale for selecting these terms and definitions was that they were agreed upon with the patient partner, MGH, as they accurately described the nature of her involvement.
Terms used in this paper include the following: patient, involvement, partnership, and co-design.
The term ‘patient’ was used because this perspective piece concerns involvement with patients as distinct from members of the public. While this term may invoke the image of an individual seeking care, in the context of involvement it also refers to individuals with experience of a health condition and its management [28], which in this case is total joint replacement surgery.
Building upon this is the definition of ‘involvement’, which is commonly accepted to refer to research done ‘with’ or ‘by’ patients rather than ‘for’, ‘to’, or ‘about’ them [30]. One specific type of involvement is the ‘patient partnership’, in which patients are embedded in various research activities at different levels and in multiple stages of the research process [1]. A particularly powerful example of a patient partnership is the Research Buddy program, which has been described previously as a program in which a doctoral student works closely with a patient throughout their project [13] and which was also described and reflected upon later in this perspective piece in the section ‘Method of Patient Involvement throughout MD–PhD program’.
Finally, the term ‘co-design’ was used in this piece to describe the active, voluntary process of researcher and patient partner working together on the design of a PhD project and its constituent studies, as this terminology is appropriate for the timeframe in which doctoral studies typically take place [22, 32,33,34,35].
Theoretical underpinnings
Theory development was not an aim of this perspective piece. However, it is important to describe the theoretical underpinnings of the patient involvement program.
There are three main approaches to patient involvement in research: epistemological, consequentialist, and emancipatory or moral [33, 36, 37]. The epistemological approach recognises the fact that patients have lived experience of a health condition that can be of benefit to the research by broadening the perspective of researchers. The emancipatory approach recognises that patients have a right to be involved in publicly funded research that impacts upon them or the care they receive, and consequently researchers have a responsibility to involve patients. Finally, the consequentialist argument states that involvement can improve the design, conduct, and reporting of research.
Primarily, the emancipatory argument motivated the formation of the involvement program described in this perspective piece. Furthermore, patient involvement can lead to more ethically sound research [38], and this was recognised in the work reflected upon in this piece as MGH assisted DG in the ethics application process for the PhD research projects through document review. However, it was also recognised that the quality and relevance of research conducted at the research centre could be improved through involvement of a patient with lived experience of joint replacement surgery. Therefore, the consequentialist and epistemological arguments also underpinned the involvement program.
Recent literature has also advocated for a pragmatic approach to patient-oriented research by utilising patient involvement to ultimately improve health outcomes for affected individuals [39]. These authors recognised that achieving such impact is a complex task and, as such, mixed methods approaches to research are encouraged to harness the strength of both quantitative and qualitative research methodologies. This is particularly relevant to the current perspective piece, which describes patient involvement in a mixed methods PhD project on a topic of clinical relevance aimed to improve outcomes for patients.
Finally, the word ‘perspective’ was intentionally selected instead of the term ‘representative’ because it is unrealistic and unfair to ask a single patient with their own unique experience to generalise their experience to represent that of all patients with the same health condition. Rather, patient involvement was incorporated to broaden the perspective of the researchers while they co-designed research with the patient [36, 40].
Context
A detailed description of the context in which the patient involvement program took place facilitates comparison to one’s own context such that the potential applicability of a framework and experience can be considered in detail [6, 7]. This is not intended to limit the generalisability of the findings reflected upon in this perspective piece. Rather, the specific context in which the patient involvement partnership and activities took place is described in detail such that readers can accurately compare this context to their own.
The patient involvement program described in this perspective piece took place in an Australian National Health and Medical Research Council Centre for Research Excellence for Total Joint Replacement, seeking to Optimise Patient oUtcomes and Selection for Total Joint Replacement (OPUS). OPUS is embedded in a hospital in Melbourne, Victoria, which is a tertiary referral centre for total joint replacement (TJR), providing care for people from a broad range of geographical regions, socioeconomic backgrounds, and cultures [41]. OPUS brings together clinician-researchers with backgrounds in orthopaedic surgery, nursing, and physiotherapy, as well as trialists, epidemiologists, health economists, statisticians and qualitative researchers. It comprises researchers at various stages of their careers, including graduate research students.
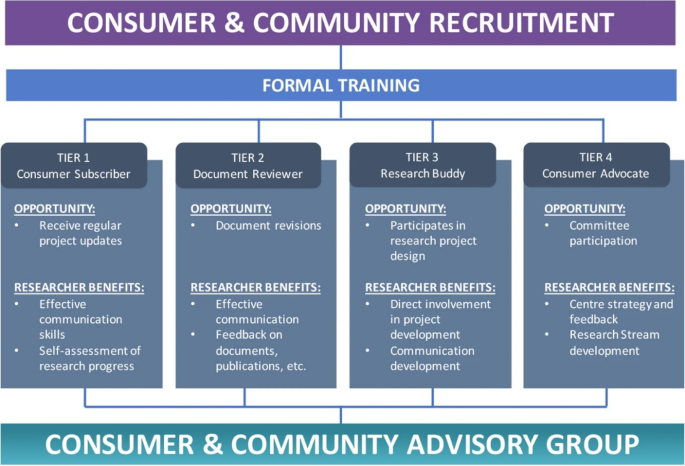
The patient involvement program at OPUS was developed and launched in 2020 and has been described previously [42]. The program involves a four-tiered framework of involvement (see Fig. 1). Table 3 of this prior publication also outlines the remuneration fee schedule according to which MGH was remunerated for her time. This prior publication used the term ‘consumer’, therefore, for consistency, this terminology is used below when describing the framework.
This Figure was made available under the Creative Commons Attribution 4.0 (http://creativecommons.org/licenses/by/4.0/).
The Consumer and Community Advisory Group (bottom of Fig. 1) oversees the operations of the CCI program. It is chaired by a consumer (MGH, co-author on this paper).
This perspective piece details the experience of a Research Buddy, Tier 3 as shown in Fig. 1, and an MD–PhD candidate at OPUS. An MD–PhD candidate is a medical student who takes time out from their clinical studies to complete a PhD. The details of this Research Buddy program are described in the next section.
Methods
Method of patient involvement throughout MD–PhD program
The Research Buddy program reflected upon in this piece took the approach of broadening the perspective of the research team by including the voice a patient with lived experience of a health condition [43]. This is distinct from an approach which would seek to achieve representativeness from one patient partner on behalf of all patients with a shared lived experience, which would be unrealistic [31, 44].
The focus of this piece is on the Research Buddy partnership comprising authors DG and MGH. In this section, DG and MGH reflected upon their expectations prior to the commencement of the Research Buddy program and how their actual experience played out.
DG was a final-year postgraduate medical student in 2019, carrying out a research project on risk factors for readmission following total knee replacement (TKR) surgery. The opportunity arose to enrol in a PhD to expand upon this work, which led to the deferment of DG’s final year of medical school in order to complete a PhD as part of an MD–PhD program [45]. Building upon the research into risk factors for readmission following TKR, the PhD centred around the development of a clinical risk prediction tool utilising machine learning. DG, along with his PhD supervisors (authors MD, SB, and PC) felt that the strong clinical focus of the MD–PhD program would position it well for close involvement with a Research Buddy. MGH had previously participated, as a subject, in a study conducted by MD and PC investigating the impact of a mindfulness program on recovery post-surgery for patients undergoing total hip replacement (THR) [46]. MGH reached out to MD regarding the findings of the mindfulness study, coincidentally while MD was establishing the patient involvement framework at OPUS and seeking volunteers for the Research Buddy role. MGH subsequently embraced the opportunity and volunteered to be DG’s Research Buddy approximately 6 months after the official commencement of the PhD. MGH decided to take part specifically in DG’s project because she wanted to learn more about research, had a keen interest in artificial intelligence and machine learning and its application to lower limb joint replacement surgery, and has had a lifelong interest and involvement in academia. This presented a tangible opportunity to make a substantial impact on the direction of the research and cement its clinical, stakeholder-focused approach, and MGH felt her interests and experience aligned most closely with DG’s project [47].
During this formative stage of the PhD project, MGH and DG worked together to define the scope and structure of the Research Buddy program. There was a shared expectation of open-ended discussions and regular contact with updates on the research. Neither DG nor MGH had been involved in such a patient involvement partnership before, therefore they did not have rigid expectations about the manner in which the partnership would unfold. It was decided that regular monthly meetings would facilitate the development of rapport, ongoing discussion of ideas for the PhD project and opportunities for involvement its constituent studies, and the opportunity for regular updates on progress.
The first part of DG’s PhD project discussed in these meetings was the systematic review, which was in the manuscript drafting stage and benefited greatly from MGH’s input on the clarity of the language [48, 49] and ensuring the pertinence and importance of the topic were clearly communicated. Patient involvement in systematic reviews has been reported extensively in the literature [32, 50,51,52], and it was an opportunity for MGH to gain familiarity with the topic at the foundation of the PhD project. More broadly, the regular monthly meetings were critical in defining the overall scope and purpose of the PhD project. Specifically, during the early stages it was difficult to determine how to make the research more clinically relevant. MGH had a background in occupational therapy and medical anthropology and had previously been involved in qualitative interview projects in which she interviewed participants and coded transcripts. The idea of a qualitative study exploring the views of TKR patients on the use of artificial intelligence and machine learning for risk prediction in shared clinical decision-making was already being developed, and MGH felt this was a fitting opportunity to be involved given her personal journey as a patient and her prior academic and research experience. MGH was involved as a co-author on the qualitative study and made a substantial contribution by pilot-testing the interview guide, contributing to the reflexive thematic analysis, and preparing and editing the manuscript for publication.
Next, MGH reviewed all of the ethics approval application documents for the studies comprising DG’s PhD project. Not only did this improve the clarity of the documents in a similar way to MGH’s review of the systematic review manuscript, but it also led to the development of more ethically conscious research by incorporating a patient’s perspective into the application [38] and helped DG to clarify the way these studies related to one another and formed a cohesive program of research.
Beyond the specific elements in which MGH made a direct contribution, DG’s reflection upon the stakeholder involvement in the form of the Research Buddy program prompted a shift in focus from machine learning to enhance predictive performance of the TKR readmission prediction model, to stakeholder involvement and engagement in the whole process of developing clinical risk prediction models, including not only patients but also clinicians [29, 53]. In light of this, thanks to MGH’s Research Buddy input, DG designed the PhD project with stakeholder involvement embedded throughout.
At the time of writing this perspective piece, the main studies of the PhD project had been completed and DG was in the final stages of thesis write-up following feedback provided by MGH on the first full draft. The overall focus of the PhD project is on co-design with stakeholders, inspired by the Research Buddy partnership with MGH and cemented by exploration of the literature [24, 54,55,56].
The PhD project centres around the development of a machine learning model to predict 30-day readmission [57] in people undergoing TKA surgery years [58, 59] for osteoarthritis (OA) [60], co-designed with clinicians and patients [61]. The project is divided into four components, each comprising at least one main study. While MGH’s involvement in the project overall has been outlined above, what follows is a brief description of each part of the PhD project as well as the level [29] and phase [62] at which MGH was involved. While a high level of involvement was aimed at from the early phases of each part of the PhD project, it is recognised that higher levels of involvement are not automatically more desirable [27] and, since some aspects of the project were already underway when MGH and DG commenced the Research Buddy program, flexibility and responsiveness to the realities of the circumstances were of utmost importance in deciding with MGH at what level she wanted to be involved.
-
1.
Identify and appraise risk factors for 30-day readmission following TKA.
-
a.
As previously mentioned, MGH was involved in reviewing the final draft of the systematic review manuscript [63] and provided feedback on the clarity of the language. The next stage of this part of the project involved a modified Delphi and focus group study involving clinicians who care for TKR patients to gain their insight on the clinical relevance of risk factors identified in the systematic review and meta-analysis, as well as giving them the opportunity to suggest novel risk factors which had not been identified in prior literature. MGH helped by observing the pilot test of the focus group and providing feedback on its structure and conduct. This study has also been published [64].
-
i.
Level = Involve
-
ii.
Phase = Design and preparation, dissemination
-
i.
-
a.
-
2.
Develop and internally validate the model.
-
a.
The findings of the systematic review and meta-analysis, as well as the Delphi and focus group study, were utilised alongside machine learning to develop a clinically applicable risk prediction tool for 30-day readmission following TKR which harnessed both clinical reasoning and statistical learning techniques. The manuscript is currently under review in an academic journal. MGH and DG discussed the project in their regular meetings as DG designed the study, particularly the purpose of the predictive tool and the way in which it could be used in the live clinical setting to facilitate shared clinical decision-making between patient and surgeon. and MGH reviewed several drafts of the manuscript ahead of submission for publication.
-
i.
Level = Involve
-
ii.
Phase = Dissemination
-
i.
-
a.
-
3.
Explore the understanding of AI for risk prediction in shared clinical decision-making among people with TKA.
-
a.
This is the qualitative semi-structured interview study, involving interviews with 20 total knee replacement patients, in which MGH had the most direct involvement, being included as a co-author. Patient and stakeholder involvement in qualitative studies is a longstanding practice [5, 7, 65,66,67,68,69]. MGH was involved in refining the research question and interview guideline through two rounds of pilot-testing. MGH was also actively involved in the reflexive thematic analysis and in drafting the manuscript for submission to an academic journal, where it is currently under review.
-
i.
Level = Collaborate
-
ii.
Phase = Design and preparation, conduct and implementation, and dissemination
-
i.
-
a.
-
4.
Compare the performance of the predictive model to that of clinicians involved in the care of people receiving TKA surgery.
-
a.
Preliminary findings for this study have been analysed and recruitment is almost complete at the time of writing this perspective piece. MGH was involved in a similar way to the predictive model development study, however this comparison study involved recruiting clinicians to participate in a survey. MGH reviewed recruitment material and discussed with DG how to structure the online survey to ensure the instructions were clear and straightforward and the length of the survey was manageable for busy clinicians.
-
i.
Level = Involve
-
ii.
Phase = Design and preparation, dissemination
-
i.
-
a.
Method of reflection in this perspective piece
Rather than speaking for the patient, it is important for the patient to speak for themselves. Prior reflective work on patient involvement in doctoral research adopted this approach to great effect [5, 14]. This piece was therefore co-written by MGH and DG, the two members of the inaugural Research Buddy partnership at OPUS.
Pertinent elements of the GRIPP2 (Guidance for Reporting Involvement of Patients and the Public) checklist [70] were adhered to while writing this piece.
The experiential insight gained by DG and MGH through their involvement in the Research Buddy program was consolidated in regular meetings throughout DG’s PhD project. Additionally, DG and MGH conducted an informal interview using questions informed by prior literature in which patient advocates from various patient involvement programs in the UK were interviewed [9]. This interview was audio recorded and transcribed. The transcript was then analysed using reflective thematic analysis [71] in which themes were generated inductively by DG and MGH based on MGH’s responses to the questions in the interview, informed by their shared experience in the Research Buddy partnership. These themes were then synthesised and refined into lessons, inspired by prior patient involvement literature which has used thematic analysis in a similar way to generate lessons and recommendations [1, 3, 55, 62, 72, 73].
A literature search was then carried out to identify literature reviews which contained lessons from, or recommendations for, patient involvement [1, 5, 13, 15, 28, 35, 62, 74, 75]. These reviews were used to corroborate the findings from our own inductive analysis and reflection.
DG and MGH derived these lessons by reflecting primarily on their experience in the Research Buddy program. However, MGH was embedded in various other patient involvement activities at the research centre (OPUS), therefore some of these lessons will be broadly applicable beyond Research Buddy partnerships. This will depend on the patient’s desired level of involvement, and it is advised that readers are flexible in their interpretation and implementation of these findings.
Findings: lessons learned
Lesson (1) learn from experience
Don’t go in completely blind. Learn from the experience of others as much as possible. Bring in experienced patient partners, and researchers experienced in patient involvement, who can offer advice on promising avenues to explore as well as flagging those which are less likely to be fruitful. For example, as part of the Research Buddy program reflected upon in this perspective piece, Carol Vleeskens was consulted to conduct a training session attended by both MGH and DG. Carol was a co-author on the publication outline OPUS’ patient involvement framework [42] and has extensive experience in patient involvement [76]. MGH was then involved in preparing an orientation day for prospective patient partners at OPUS, which she also attended.
Another powerful resource is published work on the experience and appraisal of other researcher-patient partnerships sharing their experience [5].
This lesson is pertinent to consider both in preparation for partnering with patients in order to maximise the likelihood of success prior to launching involvement programs [8, 13, 48, 77], as well as for ongoing appraisal and improvement of existing programs based on patient partner feedback [1].
Lesson (2) tailor the program
A one-size-fits-all approach does not work. Patient involvement programs should be bespoke. They should be tailored to the unique context of the research centre engaging the patients [5, 78]. Learning from the work of others is critical for generating ideas about how one’s own program could take shape [78], but it is equally important to modify these programs so they are fit for purpose.
Tailoring the patient involvement program has been emphasised in prior literature as a critical component of fruitful involvement through the translation of general principles into context-specific systems and actions [4, 5, 8, 15, 35, 74].
Lesson (3) get involved early
If possible, involve patients in discussions where ideas are generated for research projects. Allow ample time to include patients on ethics applications and governance approvals to ensure they can make a meaningful contribution to the research.
Integrating patient involvement in earlier stages of research helps to facilitate truly patient-led research. Based on the experience outlined in this perspective piece, there are two main ways in which this can be done. First, patient involvement can be embedded in the ongoing work of established research centres as well as the commencement of work at new research centres in order to identify research priorities and steer the direction of research activities [4, 7]. Second, in the style of Research Buddy partnerships, patients can be partnered with PhD students in order to co-design the program of research in a meaningful way by embedding engagement and co-design at the beginning of the student’s project [19]. It is not difficult to imagine a pipeline in which researchers collaborate with patients to generate ideas and then these ideas are translated into PhD research projects with patients.
Having patients engaged at these different stages of idea-generation and research project design could have the added benefit of anticipating and addressing challenges likely to arise in complex research projects such that solutions can be suggested proactively [29].
Early involvement has been demonstrated or recommended numerous times in a diverse range of contexts and for different levels of involvement [5, 7, 15, 28, 35, 43, 75].
Lesson (4) embrace uniqueness
Patients bring a wealth of unique experience. They should be treated as individuals with their own unique characteristics, experience, and insight [79]. Embrace their unique skills and experience. Throughout regular meetings and correspondence, various aspects of the patient’s experience may enable them to bring a unique perspective to the research which researchers could not have anticipated.
Embracing uniqueness of patient partners has been found to encourage more inclusive stakeholder involvement by demonstrating a flexibility in the researchers and responsiveness to the individual needs, experiences, and preferences of people seeking to be involved [1, 5, 35, 48, 77].
Lesson (5) meet regularly
Meet regularly, right from the start, even in the absence of a clear idea of what to do in terms of exactly how the patient will be involved. The level and nature of involvement can be collaboratively defined through these regular meetings.
Be open to a variety of forms the patient’s involvement could take and be willing to engage in some trial and error. Some ideas will not pan out, and that is just part of the process. Be willing to persevere and adapt. For example, during the qualitative study conducted as part of DG’s PhD project, MGH attempted cross-coding of the qualitative interview transcripts using the coding framework being developed by DG and author SB. However, MGH found that her style of coding based her prior experience was not functioning as well as it had for previous projects. DG and MGH attempted different styles and continued to discuss their findings in their regular monthly meetings, however ultimately this cross-coding exercise was abandoned as it was becoming quite convoluted and MGH felt more comfortable discussing the transcripts and contributing to the discussions around the development of themes through reflexive thematic analysis.
Both DG and MGH had an openness that resulted in these meetings being fruitful beyond the pragmatic aspects of setting the research agenda and tracking progress; they were critical in building rapport. Even when there were no specific research updates, the regular meeting time was maintained and utilised for an opportunity to discuss topics related to the research.
Regular meetings are a critical component of impactful patient involvement programs reported in the literature [15, 35, 62, 75] as they facilitates ongoing communication, continuous feedback, and strong rapport-building as expanded upon in Lesson 6.
Lesson (6) build rapport
Establishing trust and rapport is critical to the success of the program. Put in the time. Create an environment in which frank and open discussions are encouraged. Follow through on action items from meetings and email discussions. Respect the patient’s autonomy while supporting them in their role. Listen to the patient and take their feedback into account. Demonstrate to the patient how their input is influencing the research. This designated time to meet and talk openly was critical for building rapport, which is arguably the most important component of patient involvement to increase the likelihood of success [37, 80]. The centrality of rapport to the overall success of patient involvement in research is reflected in the large volume of diverse literature recognising its importance [1, 5, 17, 28, 30, 35, 62, 73, 75, 81,82,83,84].
Lesson (7) ensure mutual benefit
Patients are more engaged when there is something in it for them. Ensure the interaction is mutually beneficial. For example, patients might learn more about their condition, or the condition of those they care for, through the patient involvement program [85].
This pragmatic aspect of involvement has been recognised in the literature detailing meaningful and impactful involvement activities that recognise the importance of ensuring the involvement benefits the research, the researchers, and the patient partners themselves [1, 19, 35, 62, 75, 83].
The nature of a successful patient involvement program, in which there is rapport, mutual respect, and open discussion, is associated with a certain degree of passive learning simply by the two parties being in contact with one another [85]. Without any specific educational component to the program, regular and open communication regarding a common focus in the context of a research centre or program can have the effect of educating the patient about research [53], and the researcher about the patient’s lived experience of their condition [72, 86]. Patients may also learn more about their own condition, its management, and current research being done to further understanding about it [85].
Lesson (8) broad involvement
It is beneficial to have the patient embedded in the broader patient involvement activities at the research centre, not just the Research Buddy (or equivalent) program. This may enable the patient to participate in broad discussions about current research and setting the direction of future research.
This is important for contextualising the Research Buddy’s work and enhancing their understanding of the way in which it fits into the broader body of work being conducted at the research centre in which the program is taking place. This is not to say patient partners should be stretched beyond their limit, but rather that they are not forced to limit themselves to one project and instead are encouraged to broaden their perspective through opportunities to be involved in varying capacities in other research projects and activities depending on their interests and capacity [28, 35, 82].
Lesson (9) regularly reflect and review
It is not enough to have good intentions. Having a framework is also critical, but the patient’s role may evolve over time and ethical considerations related to their involvement also evolve. It is easy for the patient’s role to devolve into tokenism despite the best intentions [22]. Regular meetings incorporating feedback from patient partners on their involvement in the research are critical to ensuring the patient’s role as partner in the research is respected and maintained. This requires transparency on the part of the researcher regarding how the patient’s involvement is influencing the research. Researchers must also be open to feedback from patients that may indicate they are not satisfied with their involvement and adjustments to their involvement may be required.
One potential way in which the patient partnership can break down is for researchers to treat them less like partners in the research process and more like participants in quasi-qualitative research by simply calling upon patients to complete surveys or answer questions as if in a qualitative interview. This is problematic because patient involvement in research is distinct from qualitative research and must be treated as such, but vigilance and effort is required to maintain the boundaries between the two [22].
Beyond this specific problem, regular reflection and review are broadly applicable to every aspect of patient involvement to ensure the patient’s involvement is meeting expectations as these expectations evolve throughout the research project alongside changes in the patient’s capacity and interests.
Regular review is crucial to ensure ethical principles are being adhered to at all times as the patient’s role evolves. Circumstances change, both intentionally and unintentionally, so it is critical to have discussions specifically dedicated to reviewing the patient’s role and ensuring they are still meaningfully involved and ethical principles are adhered to [87]. There may be occasions on which the patient’s preconceived ideas need to be discussed and worked through.
Building upon the other lessons outlined in this perspective piece, rapport based on mutual respect, coupled with regular meetings, provide the structure and nurture the culture necessary to facilitate continuous feedback as well as regular designated touch points at which reflection and review can take place [5, 13, 28, 35, 62, 74, 75, 83, 87].
Discussion
Summary of findings
In this perspective piece, a patient partner with lived experience of total joint replacement surgery (MGH) and a MD–PhD candidate (DG) reflected on their experience in a Research Buddy program throughout a PhD project. Their aim was to provide experiential insight such that other researchers and patient partners can learn from their experience as they develop and refine their own patient involvement programs. Inductive thematic analysis was applied to the transcript of an informal interview carried out between DG and MGH, informed by reflections from regular meetings throughout their Research Buddy partnership. Through this process, nine lessons were synthesised from their experience: Learn from experience, Tailor the program, Get involved early, Embrace uniqueness, Meet regularly, Build rapport, Ensure mutual benefit, Broad involvement, and Regularly reflect and review. These lessons were corroborated by published literature, and rapport was found to be the most important factor in successful patient involvement. Each of the remaining lessons either stem from and/or contribute to the development of rapport.
Contribution to the literature
To the best of the authors’ knowledge, this is the first perspective piece on patient partnership in a MD–PhD program. This builds upon prior work highlighting the positive impact of partnering medical students with patients to further their clinical education and broaden their perspective, while empowering patients through the opportunity to learn more about their own health condition and health service experience while sharing their experiential insight with future clinicians in a formative stage of their career [88, 89]. It builds upon this by demonstrating how MD–PhD students, being situated at the interface between the research and clinical domains, can partner with patients in a fruitful way that has a meaningful impact upon their clinical development while also influencing the design, conduct, and reporting of the research. This was particularly noteworthy during DG’s PhD journey, most of which was undertaken during lockdowns imposed in light of the COVID-19 pandemic. Regular contact with MGH was, for long periods of time, DG’s only patient interaction and therefore was crucial to maintaining patient contact [88] from a clinical perspective as well as working with MGH to develop solutions to convert various research activities to be entirely online [15, 16].
Through regular meetings based on mutual trust and respect, largely comprising open discussion especially when there were no major research progress updates, building rapport was prioritised from the outset of the Research Buddy program. Since MGH and DG had the freedom to define the scope and nature of the program, they were able to emphasise the importance of this reciprocal relationship both through conversation as well as through document review, providing comments alongside DG’s PhD supervisors, which enabled MGH to provide a critical perspective on DG’s work. With strong rapport as the foundation and critical feedback encouraged throughout the research journey, MGH’s role as ‘critical friend’ was characterised and strengthened [10, 17].
This perspective piece also builds upon exemplary prior work reporting a Research Buddy program [13] by being co-authored with the Research Buddy. This perspective piece conveyed lessons learned through a program which drew on elements of embedded consultation [5, 13] and demonstrated flexibility in the level of involvement in various aspects of the PhD project in response to changes in MGH’s interests and capacity throughout the partnership [16].
Furthermore, while the PhD project reflected upon in this perspective piece did not strictly pertain to a digital health innovation nor to a full translational research innovation, a predictive model was developed which was intended for use in the clinical setting for shared clinical decision-making in orthopaedics. Therefore, MGH’s involvement throughout the project was critical for in laying a solid foundation for the development of a clinically relevant digital health innovation [90, 91] primed for translation into the clinical setting [27] in orthopaedics, which is a clinical discipline in which patient involvement is gaining recognition and appreciation [26].
Implications of findings
The most impactful implication of the findings of this paper is that the description of the context in which the researcher-patient partnership took place, coupled with the co-authored lessons learned and experiential insight [3] offered through reflection by both members of the Research Buddy partnership, can be utilised by others [92, 93]. Learning through experience and reporting these lessons along with a detailed description of the context in which they were learned [6, 18] is an impactful outcome of its own [92]. This enables readers to inform their own involvement efforts, both when establishing involvement programs and refining them.
Through involvement, patients like MGH learn more about their health condition as well as the research that goes into the developments in management approaches and the way in which patients experience the healthcare system [85].
It is hoped that these findings can be of assistance to ongoing [11, 12] and future patient involvement efforts to bring researchers and patients onto the same page from the outset of research projects [49].
Future directions
Evaluating impact
The question of how best to evaluate impact of patient involvement is longstanding and complicated [48, 77]. There have been many attempts over the years to answer this question [11, 12, 23, 73, 78, 79, 94,95,96,97,98,99], but it appears there is no single best approach to measuring the effectiveness, success, or impact of involvement [100], and perhaps such a measure will never exist. However, ongoing efforts to develop and validate consistent patient involvement evaluation measures are encouraged to facilitate the comparison of different involvement strategies and gain some indication of which might be the most promising in a given context, when resources are limited and ought to be allocated to the most promising strategy [101].
Exploring patient involvement in decision-making non-research patient involvement and empowerment
The research project reflected upon in this perspective piece centred around the development of a risk prediction tool intended to be used in the process of shared clinical decision-making. This perspective piece explored patient involvement in clinically-oriented research being carried out by a MD–PhD candidate, which positions it well for ongoing work exploring the role patients play in the shared clinical decision-making process [102,103,104], particularly as it pertains to the use of decision aids including predictive tools.
Further reading
Further reading is recommended for those seeking to expand their knowledge of different types of patient involvement in varied contexts in order to better understand how to initiate, or improve, their own patient involvement programs [32, 40, 105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148].
Strengths and limitations
Strengths of this perspective piece include the consistent and frequent contact between DG and MGH throughout the course of the PhD program, which facilitated widespread and deep involvement in various aspects of the research project and its constituent studies. This also facilitated continuous feedback and reflection through open discussion, which laid the foundation for the writing of this piece.
One potential limitation is that MGH may be regarded as a ‘professional’ patient partner, given her academic background. This may raise concerns about whether her perspective as a patient is diminished by her familiarity with academia and the research process. This was certainly an important consideration, especially considering MGH’s involvement spanned over 3 years and involved close and regular contact with DG. However, it was not a concern because regardless of how familiar MGH becomes with the research process, she will never lose her perspective as a patient who has lived experience of total joint replacement surgery [149].
Conclusions
In this perspective piece, a patient and a medical student completing a PhD reflected upon their experience co-designing a Research Buddy partnership within a patient involvement program. To the authors’ knowledge, this is the first time such a partnership has been written about within the context of a MD–PhD program, which is uniquely placed at the interface between the research and clinical domains. A series of nine lessons was identified and presented to inform readers seeking to develop or enhance their own patient involvement programs: learn from experience, tailor the program, get involved early, embrace uniqueness, meet regularly, build rapport, ensure mutual benefit, broad involvement, regularly reflect and review. Researcher-patient rapport is foundational to all other aspects of the patient’s involvement.
Availability of data and materials
Not applicable.
Abbreviations
- PhD:
-
Doctor of philosophy
- MD:
-
Doctor of medicine
- GRIPP2:
-
Guidance for reporting Involvement of Patients and the Public
- OPUS:
-
OPtimise Patient oUtcomes and Selection for Total Joint Replacement
- TKR:
-
Total knee replacement
- THR:
-
Total hip replacement
References
McCarron TL, Clement F, Rasiah J, Moran C, Moffat K, Gonzalez A, et al. Patients as partners in health research: a scoping review. Health Expect. 2021;24(4):1378–90.
Holzer JK, Ellis L, Merritt MW. Why we need community engagement in medical research. J Investig Med. 2014;62(6):851–5.
Staley K, Doherty C. It’s not evidence, it’s insight: bringing patients’ perspectives into health technology appraisal at NICE. Res Involv Engagem. 2016;2(1):1–12.
Manafò E, Petermann L, Vandall-Walker V, Mason-Lai P. Patient and public engagement in priority setting: a systematic rapid review of the literature. PLoS ONE. 2018;13(3):e0193579.
Dawson S, Ruddock A, Parmar V, Morris R, Cheraghi-Sohi S, Giles S, et al. Patient and public involvement in doctoral research: reflections and experiences of the PPI contributors and researcher. Res Involv Engagem. 2020;6(1):1–13.
Staley K, Buckland SA, Hayes H, Tarpey M. ‘The missing links’: understanding how context and mechanism influence the impact of public involvement in research. Health Expect. 2014;17(6):755–64.
Ní Shé É, Cassidy J, Davies C, De Brún A, Donnelly S, Dorris E, et al. Minding the gap: identifying values to enable public and patient involvement at the pre-commencement stage of research projects. Res Involv Engagem. 2020;6(1):1–10.
Concannon T, Grant S, Welch V. Practical guidance for involving stakeholders in 626 health research. J Gen Intern Med. 2018;34:458–63.
Bec Hanley KS. User and carer involvement: sharing our experience. London: London Multicultural Community Association (LMCA); 2005.
Tomlinson J, Medlinskiene K, Cheong V-L, Khan S, Fylan B. Patient and public involvement in designing and conducting doctoral research: the whys and the hows. Res Involv Engagem. 2019;5(1):1–12.
Horgan F, Lennon O, Hickey A, Sorensen J, Kroll T, McCartan D, et al. A protocol to evaluate the impact of embedding Public and Patient Involvement in a structured PhD programme for stroke care. Front Rehabilit Sci. 2022. https://doi.org/10.3389/fresc.2022.877598/pdf.
Foley L, Kiely B, Croke A, Larkin J, Smith SM, Clyne B, et al. A protocol for the evaluation of the process and impact of embedding formal and experiential public and patient involvement training in a structured PhD programme. J Multimorb Comorb. 2021;11:26335565211024790.
Coupe N, Mathieson A. Patient and public involvement in doctoral research: impact, resources and recommendations. Health Expect. 2020;23(1):125–36.
Troya MI, Chew-Graham CA, Babatunde O, Bartlam B, Higginbottom A, Dikomitis L. Patient and public involvement and engagement in a doctoral research project exploring self-harm in older adults. Health Expect. 2019;22(4):617–31.
Manikandan M, Foley K, Gough J, Harrington S, Wall É, Weldon F, et al. Public and patient involvement in doctoral research during the COVID-19 pandemic: reflections on the process, challenges, impact and experiences from the perspectives of adults with cerebral palsy and the doctoral researcher. 2022.
Rees L, Sherwood M, Shields N. Consumer engagement in doctoral research–what difference does it make? Spinal Cord. 2022. https://doi.org/10.1038/s41393-022-00871-1.
Jones B, Hunt A. Collaboration between doctoral researchers and patient research partners: reflections and considerations. Re All. 2022;6(1):1–9.
Staley K. Changing what researchers’ think and do’: is this how involvement impacts on research? Res All. 2017. https://doi.org/10.18546/RFA.01.1.13.
Forsythe LP, Carman KL, Szydlowski V, Fayish L, Davidson L, Hickam DH, et al. Patient engagement in research: early findings from the Patient-Centered Outcomes Research Institute. Health Aff. 2019;38(3):359–67.
Tierney E, McEvoy R, O’Reilly-de Brún M, de Brún T, Okonkwo E, Rooney M, et al. A critical analysis of the implementation of service user involvement in primary care research and health service development using normalization process theory. Health Expect. 2016;19(3):501–15.
Telford R, Beverley CA, Cooper CL, Boote JD. Consumer involvement in health research: fact or fiction? Br J Clin Gov. 2002;7(2):92–103.
Locock L, Boaz A. Drawing straight lines along blurred boundaries: qualitative research, patient and public involvement in medical research, co-production and co-design. Evid Policy. 2019;15(3):409–21.
Barber R, Boote JD, Parry GD, Cooper CL, Yeeles P, Cook S. Can the impact of public involvement on research be evaluated? A mixed methods study. Health Expect. 2012;15(3):229–41.
Boote J, Baird W, Beecroft C. Public involvement at the design stage of primary health research: a narrative review of case examples. Health Policy (Amsterdam, Netherlands). 2010;95(1):10–23.
Madden M, Speed E. Beware zombies and unicorns: toward critical patient and public involvement in health research in a neoliberal context. Front Sociol. 2017;2:7.
Owyang D, Bakhsh A, Brewer D, Boughton OR, Cobb JP. Patient and public involvement within orthopaedic research: a systematic review. JBJS. 2021;103(13):e51.
Van der Scheer L, Garcia E, van der Laan AL, van der Burg S, Boenink M. The benefits of patient involvement for translational research. Health Care Anal. 2017;25:225–41.
Manafo E, Petermann L, Mason-Lai P, Vandall-Walker V. Patient engagement in Canada: a scoping review of the ‘how’and ‘what’of patient engagement in health research. Health Res Policy Syst. 2018;16(1):1–11.
Bammer G. Key issues in co-creation with stakeholders when research problems are complex. Evid Policy. 2019;15(3):423–35.
Carroll P, Dervan A, Maher A, McCarthy C, Woods I, Kavanagh R, et al. Applying patient and public involvement in preclinical research: a co-created scoping review. Health Expect. 2022;25(6):2680–99.
Ward PR, Thompson J, Barber R, Armitage CJ, Boote JD, Cooper CL, et al. Critical perspectives on ‘consumer involvement’in health research: epistemological dissonance and the know-do gap. J Sociol. 2010;46(1):63–82.
Boden C, Edmonds AM, Porter T, Bath B, Dunn K, Gerrard A, et al. Patient partners’ perspectives of meaningful engagement in synthesis reviews: a patient-oriented rapid review. Health Expect. 2021;24(4):1056–71.
Greenhalgh T, Hinton L, Finlay T, Macfarlane A, Fahy N, Clyde B, et al. Frameworks for supporting patient and public involvement in research: systematic review and co-design pilot. Health Expect. 2019;22(4):785–801.
Osborne SP, Radnor Z, Strokosch K. Co-production and the co-creation of value in public services: a suitable case for treatment? Public Manag Rev. 2016;18(5):639–53.
Bird M, Ouellette C, Whitmore C, Li L, Nair K, McGillion MH, et al. Preparing for patient partnership: a scoping review of patient partner engagement and evaluation in research. Health Expect. 2020;23(3):523–39.
Boote J, Baird W, Sutton A. Public involvement in the systematic review process in health and social care: a narrative review of case examples. Health Policy (Amsterdam, Netherlands). 2011;102(2–3):105–16.
Gregory S, Wells K, Forsyth K, Latto C, Szyra H, Saunders S, et al. Research participants as collaborators: background, experience and policies from the PREVENT Dementia and EPAD programmes. Dementia. 2018;17(8):1045–54.
Staley K, Minogue V. User involvement leads to more ethically sound research. Clin Eth. 2006;1(2):95–100.
Allemang B, Sitter K, Dimitropoulos G. Pragmatism as a paradigm for patient-oriented research. Health Expect. 2022;25(1):38–47.
Goel N. Enhancing patient research partner engagement: Research in psoriatic arthritis. Best Pract Res Clin Rheumatol. 2021;35(2):101685.
Gould D, Thuraisingam S, Shadbolt C, Knight J, Young J, Schilling C, et al. Cohort profile: the St Vincent’s melbourne arthroplasty outcomes (SMART) registry, a pragmatic prospective database defining outcomes in total hip and knee replacement patients. BMJ Open. 2021;11(1):e040408.
Gunatillake T, Shadbolt C, Gould D, Lam M, Hearst MG, Vleeskens C, et al. Embedding consumer and community involvement within an established research centre: moving from general recommendations to an actionable framework. Res Involv Engagem. 2020;6(1):1–8.
Kretchy IA, Okoibhole LO, Sanuade OA, Jennings H, Strachan DL, Blandford A, et al. Scoping review of community health participatory research projects in Ghana. Glob Health Act. 2022;15(1):2122304.
Thompson J, Barber R, Ward PR, Boote JD, Cooper CL, Armitage CJ, et al. Health researchers’ attitudes towards public involvement in health research. Health Expect. 2009;12(2):209–20.
Ng E, Jones AA, Sivapragasam M, Nath S, Mak LE, Rosenblum ND. The integration of clinical and research training: How and why MD–PhD programs work. Acad Med. 2019;94(5):664–70.
Dowsey M, Castle D, Knowles S, Monshat K, Salzberg M, Nelson E, et al. The effect of mindfulness training prior to total joint arthroplasty on post-operative pain and physical function: a randomised controlled trial. Complement Ther Med. 2019;46:195–201.
Staley K, Cockcroft E, Shelly A, Liabo K. ‘What can I do that will most help researchers?’A different approach to training the public at the start of their involvement in research. Res Involv Engagem. 2019;5(1):1–6.
Telford R, Boote JD, Cooper CL. What does it mean to involve consumers successfully in NHS research? A consensus study. Health Expect. 2004;7(3):209–20.
Staley K, Ashcroft J, Doughty L, Szmukler G. Making it clear and relevant: patients and carers add value to studies through research document reviews. Mental Health Soc Incl. 2016;20(1):36–43.
Boote J, Baird W, Sutton A. Involving the public in systematic reviews: a narrative review of organizational approaches and eight case examples. J Comp Effect Res. 2012;1(5):409–20.
Pollock A, Campbell P, Struthers C, Synnot A, Nunn J, Hill S, et al. Stakeholder involvement in systematic reviews: a scoping review. Syst Rev. 2018;7:1–26.
Bayliss K, Starling B, Raza K, Johansson EC, Zabalan C, Moore S, et al. Patient involvement in a qualitative meta-synthesis: lessons learnt. Res involv Engagem. 2016;2(1):1–19.
Synnot AJ, Cherry CL, Summers MP, Stuckey R, Milne CA, Lowe DB, et al. Consumer engagement critical to success in an Australian research project: reflections from those involved. Aust J Prim Health. 2018;24(3):197–203.
Pandya-Wood R, Barron DS, Elliott J. A framework for public involvement at the design stage of NHS health and social care research: time to develop ethically conscious standards. Res Involv Engagem. 2017;3(1):1–21.
McCarron TL, Moffat K, Wilkinson G, Zelinsky S, Boyd JM, White D, et al. Understanding patient engagement in health system decision-making: a co-designed scoping review. Syst Rev. 2019;8(1):1–10.
Price A, Albarqouni L, Kirkpatrick J, Clarke M, Liew SM, Roberts N, et al. Patient and public involvement in the design of clinical trials: an overview of systematic reviews. J Eval Clin Pract. 2018;24(1):240–53.
ACSQHC. Avoidable Hospital Readmissions: Report on Australian and International indicators, their use and the efficacy of interventions to reduce readmissions. Sydney: Australian Commission on Safety and Quality in Health Care. 2019.
Inacio MCS, Graves SE, Pratt NL, Roughead EE, Nemes S. Increase in total joint arthroplasty projected from 2014 to 2046 in Australia: a conservative local model with international implications. Clin Orthop Relat Res. 2017;475(8):2130–7.
Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780–5.
Carr AJ, Robertsson O, Graves S, Price AJ, Arden NK, Judge A, et al. Knee replacement. The Lancet. 2012;379(9823):1331–40.
Greenhalgh T, Jackson C, Shaw S, Janamian T. Achieving research impact through co-creation in community-based health services: literature review and case study. Milbank Q. 2016;94(2):392–429.
Harrison JD, Auerbach AD, Anderson W, Fagan M, Carnie M, Hanson C, et al. Patient stakeholder engagement in research: a narrative review to describe foundational principles and best practice activities. Health Expect. 2019;22(3):307–16.
Gould D, Dowsey MM, Spelman T, Jo O, Kabir W, Trieu J, et al. Patient-related risk factors for unplanned 30-day hospital readmission following primary and revision total knee arthroplasty: a systematic review and meta-analysis. J Clin Med. 2021;10(1):134.
Gould D, Dowsey M, Spelman T, Bailey J, Bunzli S, Rele S, et al. Established and novel risk factors for 30-day readmission following total knee arthroplasty: a modified delphi and focus group study to identify clinically important predictors. J Clin Med. 2023;12(3):747.
Gillard S, Simons L, Turner K, Lucock M, Edwards C. Patient and public involvement in the coproduction of knowledge: reflection on the analysis of qualitative data in a mental health study. Qual Health Res. 2012;22(8):1126–37.
Garfield S, Jheeta S, Husson F, Jacklin A, Bischler A, Norton C, et al. Lay involvement in the analysis of qualitative data in health services research: a descriptive study. Res Involv Engagem. 2016;2(1):1–12.
Frost J, Gibson A, Harris-Golesworthy F, Harris J, Britten N. Patient involvement in qualitative data analysis in a trial of a patient-centred intervention: reconciling lay knowledge and scientific method. Health Expect. 2018;21(6):1111–21.
Hemming L, Pratt D, Bhatti P, Shaw J, Haddock G. Involving an individual with lived-experience in a co-analysis of qualitative data. Health Expect. 2021;24(3):766–75.
Boote J, Wong R, Booth A. ‘Talking the talk or walking the walk?’A bibliometric review of the literature on public involvement in health research published between 1995 and 2009. Health Expect. 2015;18(1):44–57.
Staniszewska S, Brett J, Simera I, Seers K, Mockford C, Goodlad S, et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ (Clin Res Ed). 2017;358:j3453.
Braun V, Clarke V. Reflecting on reflexive thematic analysis. Qual Res Sport Exer Health. 2019;11(4):589–97.
Staley K, Abbey-Vital I, Nolan C. The impact of involvement on researchers: a learning experience. Res Involv Engagem. 2017;3(1):1–9.
Staniszewska S, Denegri S, Matthews R, Minogue V. Reviewing progress in public involvement in NIHR research: developing and implementing a new vision for the future. BMJ Open. 2018;8(7):e017124.
Preston J, Nafria B, Ohmer A, Gaillard S, Dicks P, West L, et al. Developing a more tailored approach to patient and public involvement with children and families in pediatric clinical research: lessons learned. Ther Innov Regul Sci. 2022;56(6):948–63.
Chudyk AM, Horrill T, Waldman C, Demczuk L, Shimmin C, Stoddard R, et al. Scoping review of models and frameworks of patient engagement in health services research. BMJ Open. 2022;12(8):e063507.
Vleeskens C. Researchers and the community in partnership- a consumer perspective. health beyond research & innovation showcase; Liverpool, New South Wales, Australia. RESEARCH4ME: Sydney Partnership for Health, Education, Research and Enterprise (SPHERE) Musculoskeletal Health Clinical Academic Group (MSK CAG); 2019.
Boote J, Barber R, Cooper C. Principles and indicators of successful consumer involvement in NHS research: results of a Delphi study and subgroup analysis. Health Policy (Amsterdam, Netherlands). 2006;75(3):280–97.
Staley K. ‘Is it worth doing?’Measuring the impact of patient and public involvement in research. Res Involv Engagem. 2015;1(1):1–10.
Staley K. An evaluation of a pilot project of PPI in research at Parkinson's UK. Edited by Parkinson's UK. Parkinson’s UK London; 2016.
Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. A systematic review of the impact of patient and public involvement on service users, researchers and communities. Pat-Pat-Center Outcomes Res. 2014;7(4):387–95.
Wilson P, Mathie E, Keenan J, McNeilly E, Goodman C, Howe A, et al. ReseArch with patient and Public invOlvement: a realisT evaluation: the RAPPORT study. Health Serv Deliv Res 2015.
Turner G, Aiyegbusi OL, Price G, Skrybant M, Calvert M. Moving beyond project-specific patient and public involvement in research. J R Soc Med. 2020;113(1):16–23.
Shippee ND, Domecq Garces JP, Prutsky Lopez GJ, Wang Z, Elraiyah TA, Nabhan M, et al. Patient and service user engagement in research: a systematic review and synthesized framework. Health Expect. 2015;18(5):1151–66.
Brett J, Staniszewska S, Mockford C, Herron-Marx S, Hughes J, Tysall C, et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. 2014;17(5):637–50.
Desborough J, Parkinson A, Lewis F, Ebbeck H, Banfield M, Phillips C. A framework for involving coproduction partners in research about young people with type 1 diabetes. Health Expect. 2022;25(1):430–42.
Bahadori S, Collard S, Williams JM, Swain I. Why do people undergo THR and what do they expect to gain: a comparison of the views of patients and health care professionals. J Pat Exp. 2020;7(6):1778–87.
Montreuil M, Martineau JT, Racine E. Exploring ethical issues related to patient engagement in healthcare: patient, clinician and researcher’s perspectives. J Bioeth Inq. 2019;16(2):237–48.
Dijk SW, Duijzer EJ, Wienold M. Role of active patient involvement in undergraduate medical education: a systematic review. BMJ Open. 2020;10(7):e037217.
Barr J, Bull R, Rooney K. Developing a patient focussed professional identity: an exploratory investigation of medical students’ encounters with patient partnership in learning. Adv Health Sci Educ. 2015;20(2):325–38.
Street J, Stafinski T, Lopes E, Menon D. Defining the role of the public in health technology assessment (HTA) and HTA-informed decision-making processes. Int J Technol Assess Health Care. 2020;36(2):87–95.
Baines R, Bradwell H, Edwards K, Stevens S, Prime S, Tredinnick-Rowe J, et al. Meaningful patient and public involvement in digital health innovation, implementation and evaluation: a systematic review. Health Expect. 2022;25(4):1232–45.
Staley K, Barron D. Learning as an outcome of involvement in research: what are the implications for practice, reporting and evaluation? Res Involv Engagem. 2019;5(1):1–9.
Staley K. The BMJ Opinion [Internet] 2018. https://blogs.bmj.com/bmj/2018/11/28/researchers-dont-know-what-theyre-missing-the-impact-of-patient-involvement-in-research/. Accessed 01 Feb 2023.
Edelman N, Barron D. Evaluation of public involvement in research: time for a major re-think? J Health Serv Res Policy. 2016;21(3):209–11.
Esmail L, Moore E, Rein A. Evaluating patient and stakeholder engagement in research: moving from theory to practice. J Comparative Effect Res. 2015;4(2):133–45.
Boivin A, L’Espérance A, Gauvin FP, Dumez V, Macaulay AC, Lehoux P, et al. Patient and public engagement in research and health system decision making: a systematic review of evaluation tools. Health Expect. 2018;21(6):1075–84.
Barber R, Beresford P, Boote J, Cooper C, Faulkner A. Evaluating the impact of service user involvement on research: a prospective case study. Int J Consum Stud. 2011;35(6):609–15.
Stergiopoulos S, Michaels DL, Kunz BL, Getz KA. Measuring the impact of patient engagement and patient centricity in clinical research and development. Ther Innov Regul Sci. 2020;54:103–16.
Pizzo E, Doyle C, Matthews R, Barlow J. Patient and public involvement: how much do we spend and what are the benefits? Health Expect. 2015;18(6):1918–26.
Russell J, Fudge N, Greenhalgh T. The impact of public involvement in health research: what are we measuring? Why are we measuring it? Should we stop measuring it? Res Involv Engagem. 2020;6(1):1–8.
Domecq JP, Prutsky G, Elraiyah T, Wang Z, Nabhan M, Shippee N, et al. Patient engagement in research: a systematic review. BMC Health Serv Res. 2014;14(1):1–9.
Fumagalli LP, Radaelli G, Lettieri E, Masella C. Patient empowerment and its neighbours: clarifying the boundaries and their mutual relationships. Health Policy (Amsterdam, Netherlands). 2015;119(3):384–94.
Hyde C, Dunn KM, Higginbottom A, Chew-Graham CA. Process and impact of patient involvement in a systematic review of shared decision making in primary care consultations. Health Expect. 2017;20(2):298–308.
Silberberg M, Martinez-Bianchi V. Community and stakeholder engagement. Prim Care: Clin Office Pract. 2019;46(4):587–94.
Nilsen ES, Myrhaug HT, Johansen M, Oliver S, Oxman AD. Methods of consumer involvement in developing healthcare policy and research, clinical practice guidelines and patient information material. Cochrane Database Syst Rev. 2006. https://doi.org/10.1002/14651858.CD004563.pub2/abstract.
Wright MT. What is participatory health research? A position paper of the international collaboration for participatory health research. Prävention und Gesundheitsförderung. 2013;8:122–31.
Staley K, Sandvei M, Horder M. A problem shared…’The challenges of public involvement for researchers in Denmark and the UK. UK: University of Southern Denmark and TwoCan Associates; 2019.
Staley K, Crowe S. More than a top 10: How James Lind alliance priority setting partnerships transform research, people and organisations. NIHR Oxford Biomedical Research Centre, UK-September. 2019.
Staley K. A series of case studies illustrating the impact of service user and carer involvement on research. London: MHRN; 2013.
Barber R, Boote JD, Cooper CL. Involving consumers successfully in NHS research: a national survey. Health Expect. 2007;10(4):380–91.
Staley K, Elliott J. Public involvement could usefully inform ethical review, but rarely does: what are the implications? Res Involv Engagem. 2017;3(1):1–17.
Muir R, Carlini JJ, Harbeck EL, Gillespie BM, Tuffaha HW, Walker RM, et al. Patient involvement in surgical wound care research: a scoping review. Int Wound J. 2020;17(5):1462–82.
Chambers E, Gardiner C, Thompson J, Seymour J. Patient and carer involvement in palliative care research: an integrative qualitative evidence synthesis review. Palliat Med. 2019;33(8):969–84.
Camelo Castillo W, Heath N, Kim J, Yang K, Ritchey ME, dosReis S, et al. Engaging stakeholders in pharmacoepidemiology research: Current state and recommendations. Pharmacoepidemiol Drug Saf. 2019;28(6):766–76.
Miah J, Dawes P, Edwards S, Leroi I, Starling B, Parsons S. Patient and public involvement in dementia research in the European Union: a scoping review. BMC Geriatr. 2019;19(1):1–20.
Dadich A, Wyer M. Patient involvement in healthcare-associated infection research: a lexical review. Infect Control Hosp Epidemiol. 2018;39(6):710–7.
Kaisler RE, Kulnik ST, Klager E, Kletecka-Pulker M, Schaden E, Stainer-Hochgatterer A. Introducing patient and public involvement practices to healthcare research in Austria: strategies to promote change at multiple levels. BMJ Open. 2021;11(8):e045618.
Cook N, Siddiqi N, Twiddy M, Kenyon R. Patient and public involvement in health research in low and middle-income countries: a systematic review. BMJ Open. 2019;9(5):e026514.
Oldfield BJ, Harrison MA, Genao I, Greene AT, Pappas ME, Glover JG, et al. Patient, family, and community advisory councils in health care and research: a systematic review. J Gen Intern Med. 2019;34:1292–303.
Guise J-M, O’Haire C, McPheeters M, Most C, LaBrant L, Lee K, et al. A practice-based tool for engaging stakeholders in future research: a synthesis of current practices. J Clin Epidemiol. 2013;66(6):666–74.
Castro EM, Van Regenmortel T, Vanhaecht K, Sermeus W, Van Hecke A. Patient empowerment, patient participation and patient-centeredness in hospital care: a concept analysis based on a literature review. Pat Educ Couns. 2016;99(12):1923–39.
Del Gaizo V, Kohlheim M. Patient engagement in pediatric rheumatology research. Rheum Dis Clin. 2022;48(1):1–13.
Harris J, Haltbakk J, Dunning T, Austrheim G, Kirkevold M, Johnson M, et al. How patient and community involvement in diabetes research influences health outcomes: a realist review. Health Expect. 2019;22(5):907–20.
Wiering B, de Boer D, Delnoij D. Patient involvement in the development of patient-reported outcome measures: a scoping review. Health Expect. 2017;20(1):11–23.
Burton A, Ogden M, Cooper C. Planning and enabling meaningful patient and public involvement in dementia research. Curr Opin Psychiatry. 2019;32(6):557–62.
Lee DJ, Avulova S, Conwill R, Barocas DA, editors. Patient engagement in the design and execution of urologic oncology research. Urologic Oncology: Seminars and Original Investigations; 2017: Elsevier.
Bethell J, Commisso E, Rostad HM, Puts M, Babineau J, Grinbergs-Saull A, et al. Patient engagement in research related to dementia: a scoping review. Dementia. 2018;17(8):944–75.
Tivey A, Huddar P, Shotton R, Cheese I, Daniels S, Lorigan P, et al. Patient engagement in melanoma research: from bench to bedside. Future Oncol. 2021;17(28):3705–16.
Davies-Teye BB, Medeiros M, Chauhan C, Baquet CR, Mullins CD. Pragmatic patient engagement in designing pragmatic oncology clinical trials. Fut Oncol. 2021;17(28):3691–704.
Harting J, Kruithof K, Ruijter L, Stronks K. Participatory research in health promotion: a critical review and illustration of rationales. Health Promot Int. 2022;37(Supplement_2):ii7–20.
Puts MT, Sattar S, Ghodraty-Jabloo V, Hsu T, Fitch M, Szumacher E, et al. Patient engagement in research with older adults with cancer. J Geriatr Oncol. 2017;8(6):391–6.
Cook T, Boote J, Buckley N, Vougioukalou S, Wright M. Accessing participatory research impact and legacy: developing the evidence base for participatory approaches in health research. Educ Act Res. 2017;25(4):473–88.
Slåtsveen R-E, Wibe T, Halvorsrud L, Lund A. Needs-led research: a way of employing user involvement when devising research questions on the trust model in community home-based health care services in Norway. Res Involvd Engagem. 2021;7(1):1–10.
Entwistle VA, Renfrew MJ, Yearley S, Forrester J, Lamont T. Lay perspectives: advantages for health research. BMJ (Clin Res Ed). 1998;316(7129):463–6.
Chalmers I. What do I want from health research and researchers when I am a patient? BMJ (Clin Res Ed). 1995;310(6990):1315–8.
Oliver S, Liabo K, Stewart R, Rees R. Public involvement in research: making sense of the diversity. J Health Serv Res Policy. 2015;20(1):45–51.
Fudge N, Wolfe C, McKevitt C. Involving older people in health research. Age Ageing. 2007;36(5):492–500.
Crockett LK, Shimmin C, Wittmeier KD, Sibley KM. Engaging patients and the public in health research: experiences, perceptions and training needs among Manitoba health researchers. Res Involv Engagem. 2019;5(1):1–11.
Parkinson B. Patient and public involvement for mental health researchers. Mental Health Pract. 2021;24(2):22–6.
Blair C, Best P, Burns P, Campbell A, Davidson G, Duffy J, et al. ‘Getting involved in research’: a co-created, co-delivered and co-analysed course for those with lived experience of health and social care services. Res Involv Engagem. 2022;8(1):1–16.
Cox R, Kendall M, Molineux M, Miller E, Tanner B. Consumer engagement in occupational therapy health-related research: a scoping review of the Australian occupational therapy journal and a call to action. Aust Occup Ther J. 2021;68(2):180–92.
Dudley L, Gamble C, Preston J, Buck D, Group EPA, Hanley B, et al. What difference does patient and public involvement make and what are its pathways to impact? Qualitative study of patients and researchers from a cohort of randomised clinical trials. PLoS ONE. 2015;10(6):e128817.
Troya MI, Bartlam B, Chew-Graham CA. Involving the public in health research in Latin America: making the case for mental health. Rev Panam Salud Publica. 2018;42:e45.
Jinks C, Carter P, Rhodes C, Taylor R, Beech R, Dziedzic K, et al. Patient and public involvement in primary care research-an example of ensuring its sustainability. Res Involv Engagem. 2016;2(1):1–12.
Wiggins A, Wilbanks J. The rise of citizen science in health and biomedical research. Am J Bioeth. 2019;19(8):3–14.
Staley K. An evaluation of service user involvement in studies adopted by the mental health research network. Methods. 2012;10(1):1–82.
Staley K. Lay REC members: patient or public? J Med Eth. 2013;39(12):780–2.
Hamilton CB, Leese JC, Hoens AM, Li LC. Framework for advancing the reporting of patient engagement in rheumatology research projects. Curr Rheumatol Rep. 2017;19:1–10.
Staley K. There is no paradox with PPI in research. J Med Eth. 2013;39(3):186–7.
Acknowledgements
We acknowledge the work of Dr Tilini Gunatillake and Dr Michelle Lam in establishing the OPUS CCI program. We also acknowledge the work of Dr Elizabeth Nelson, Claire Weeden and Lauren Patten in leading, administering, maintaining, and expanding the program. MMD is supported by a University of Melbourne Dame Kate Campbell Fellowship. PFC holds a National Health & Medical Research Council Practitioner Fellowship (GNT1154203)
Funding
No funding was received directly for this study. DG, MGH, and SB receive no funding. PFC had the following funding sources to declare: Royalties from Johnson and Johnson, Consultancy with Johnson & Johnson, Consultancy with Stryker Corportation (paid personally); Australian National Health & Medical Research Council Practitioner Fellowship (paid to institution), HCF Foundation, BUPA Foundation, St. Vincents Health Australia, Australian Research Council, (Grant support provided to institution for research unrelated to the current manuscript); Axcelda cartilage regeneration project, Patent applied for device, composition of matter and process (institution and personally). MMD had the following funding sources to declare: National Health and Medical Research Council, HCF Foundation, BUPA Foundation, St. Vincents Health Australia, Australian Research Council, (Grant support provided to my institution for research unrelated to the current manuscript).
Author information
Authors and Affiliations
Contributions
DG and MGH conceptualised the manuscript and wrote the initial draft, with PFC, MMD, and SB providing intellectual content. All authors contributed to discussions in which the recommendations were synthesised and refined. All authors contributed to revising the manuscript prior to submission, and have all reviewed and approved the final manuscript. All authors agree to be accountable for all aspects of the manuscript and will work together to ensure questions relating to the accuracy and integrity of any part of it are appropriately investigated and resolved. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
DG, MGH, and SB have no competing interests. PFC has the following to declare: Chair, Research Committee, Australian Orthopaedic Association (now completed term); Emeritus Board Member Musculoskeletal Australia. MMD has the following to declare: Chair, Australian Orthopaedic Association Research Foundation, Research Advisory Committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Gould, D.J., Glanville-Hearst, M., Bunzli, S. et al. Research Buddy partnership in a MD–PhD program: lessons learned. Res Involv Engagem 9, 4 (2023). https://doi.org/10.1186/s40900-023-00414-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40900-023-00414-9